Computational Neuropathology
(Team Leader: Daniel Amsel)
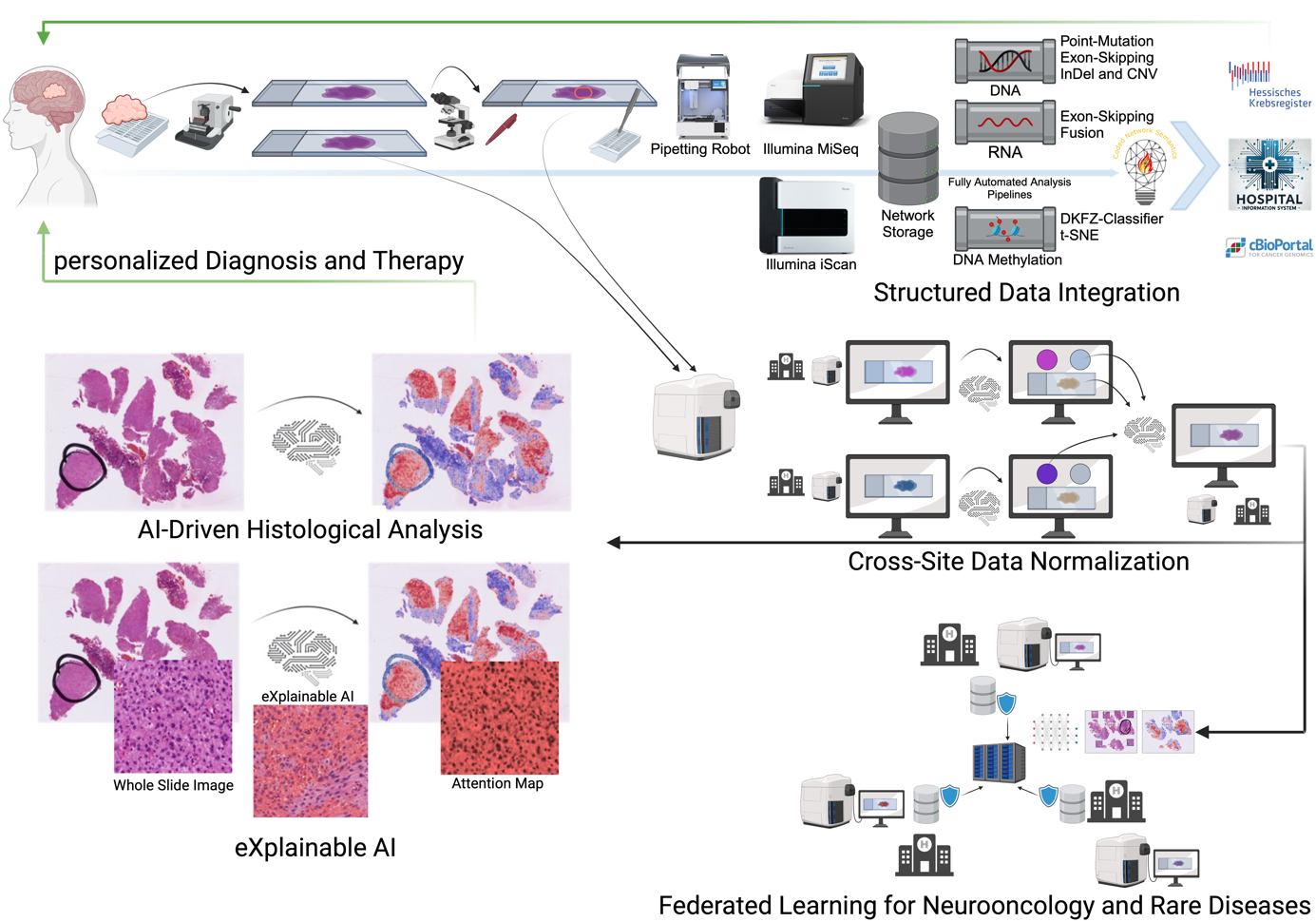
The focus area Computational Neuropathology employs advanced computational tools to address key challenges in neuropathological diagnostics and research. Together with the BMBF-funded junior research group AI-RON, we integrate bioinformatics, artificial intelligence (AI), federated learning to analyze large-scale, complex datasets in a privacy-compliant manner. By combining structured patient data with high-dimensional omics datasets and histological information, we aim to uncover actionable insights that improve diagnostic precision, therapeutic strategies, and our understanding of rare and complex diseases such as brain tumors and neuromuscular disorders. AI approaches require extensive datasets to achieve statistically meaningful results, that cannot be achieved at one site. Federated learning provides a solution by enabling collaborative machine learning without transferring sensitive patient data. This approach allows the development of robust AI models across institutions while maintaining strict compliance with privacy regulations such as GDPR, aligning with the goals of the Medical Informatics Initiative (MII). To ensure interpretability of AI models, we employ explainable AI (XAI) methods to provide clinicians with transparent and trustworthy insights, enabling the integration of these tools into clinical workflows.